UMF Corporation‘s Micrillon® microfiber, made in the USA, is setting a new standard as the first hospital-grade microfiber that can be converted into a wide range of long-lasting, reusable products, including: towels; cubicle curtains; socks; gloves; walk-off mats; reusable N95 rated face masks; and color-coded microfiber cleaning products. Leading manufacturers are using these high performance microfiber yarns to develop a new standard for materials targeting infection prevention in hospitals, hotels, cruise lines, long term care, and many other industries, according to a news release.
“The introduction of Micrillon microfiber puts an end, once and for all, to any question about recontamination related to reusable products,” said UMF Corporation CEO George Clarke. “Some manufacturers and distributors of disposable products – including cubicle curtains, microfiber mops, and wipers – have generated controversy around the risk of reusable products, such as microfiber wipers, recontaminating a patient room, even after being laundered. This illusive thinking ignores the fact that after laundering, wipers used in hospitals are immersed in an EPA-registered disinfectant before use – effectively killing bacteria and inactivating viruses.”
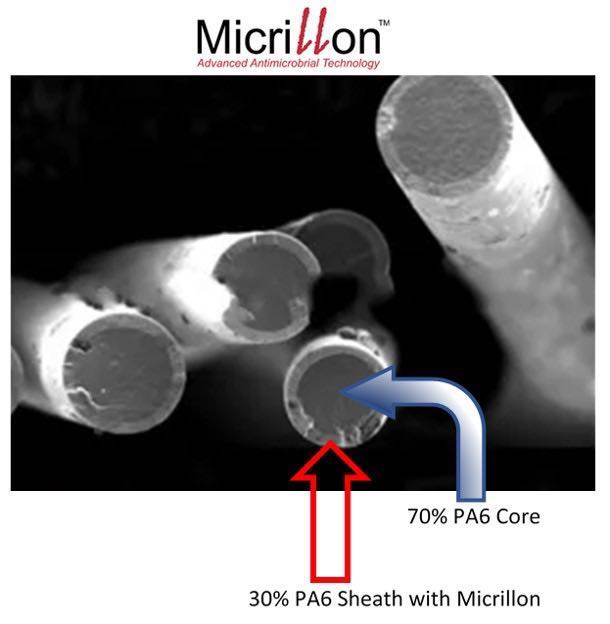
Micrillon Microfiber Yarns
Micrillon is a rechargeable polymer additive that can be incorporated into manmade fibers, films, and plastics and then charged with chlorine molecules. The Micrillon chemistry recharges for the life of the product, and will not leach into the environment. When microbes come into contact with a Micrillon surface, they are eliminated; viruses are inactivated.
UMF’s series of high-performance Micrillon yarns, including sheath and core, bicomponent segmented pie, and hollow core segmented pie, demonstrate significant antiviral properties against Human Coronavirus, which causes COVID-19, and Human Influenza A H1N1 virus in just minutes. Micrillon also demonstrates 100 percent antibacterial elimination of Staphylococcus aureus (MRSA) and E. coli 0157:H7, according to the release.
“UMF has dedicated significant time and resources developing the unrivaled Micrillon cleaning system,” added Clarke. “Micrillon products can physically remove everything from a surface and absorb it into the textile, where the disinfectant dwell time exceeds that required to inactivate and kill microbes. These durable, reusable products are cost-effective and sustainable. They significantly reduce the burden on the medical products waste stream.”
According to Dr. Mina Izadjoo, president and chief science officer at leading contract services organization Integrated Pharma Services, which conducted Micrillon’s antimicrobial efficacy testing: “We had the opportunity to test Micrillon technology and our results demonstrated significant antimicrobial activity against various pathogens. We are proud that our test and evaluation may lead to the advancement of much-needed infection control measures against hard-to-treat and drug-resistant pathogens.”
For more about the Micrillon and Klorese system, contact UMF Corporation here.














