By David Burnham, EDRO DynaWash
Water is an essential element in any laundry and the quality of water used in the laundry process can have a direct effect on the appearance and wear life of clothing and textiles.
Many industrial laundries are concerned about the hardness of the water used and because of this problem, more soap and synthetic detergents are required in hard-water laundry areas.
Water is an excellent solvent. As water moves through soil and rock, it dissolves very small amounts of the minerals it comes in contact with and holds them in solution.
The dominant causes of water hardness are calcium and magnesium salts, other dissolved metal salts, bicarbonates, silicates and sulfates.
Hardness in water increases the difficulty of soil and stain removal and can have an adverse effect on some of the chemicals used in the laundry process. It is very important that the laundry operator regularly monitor water hardness and that the operator makes decisions about changing the amount of chemicals required, installing a water softener or recharging an existing water softener.
If the removal of water hardness is not successful it can lead to “graying” in processed textiles and the increased use of chemicals. Graying is caused by re-deposition of soiling from the wash liquor. It is increased by a chemical reaction occurring between the detergent and the hardness salts producing a gray scum.
Water should be checked not just for bacteria, but for other contaminants that can potentially lead to serious problems with quality and production.
Poor management of hardness levels will lead to boiler and equipment breakdowns, exaggerated fuel costs, lost production and graying caused by the unremoved hardness neutralizing the detergent.
Hard water leaves lime scale deposits on washing and finishing equipment. The hardness breaks down in the boiler to form carbon dioxide. This then dissolves in the liquid condensate to form carbonic acid, causing corrosion in the pipework and fittings in the condensate system (including the boiler feed tank) as shown in the photo above.
Unless located in a naturally soft water area, the laundry’s water supply will have to be softened to a satisfactory level before it can be used. Before softening, the harder the water the higher its natural alkalinity will be. Only the hardness ions, not the alkalis are removed during the softening process. Softening frequently increases alkalinity levels increasing the risk of yellowing (galling).
When using highly alkaline water, good rinsing can be difficult to accomplish. In some areas where the water is very hard, the alkalinity of the raw water will need to be “soured” or neutralized. This is achieved by adding a small amount of acid to the final rinse.
Hard water causes soap to lose its effectiveness. This happens because the soap is turned into insoluble curds that have no surfactant properties to extract and suspend soil. Synthetic surfactants, many of which do not produce insoluble curds, also have their effectiveness reduced by water hardness.
When laundry water is hard it can lead to incomplete soil removal. White fabrics become gray and have a dingy appearance while colored fabrics lose their brightness.
Fabric fibers become harsh and scratchy when unremoved soil deposits are allowed to build up. The wear life of a garment can be lessened by up to 40% when washed in hard water and some fabrics may have to be discarded before wearing out.
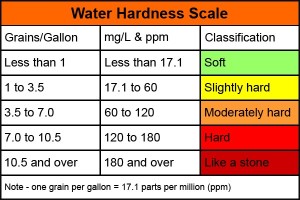
Water hardness is measured in parts per million (ppm) or grains per U.S. gallon (gpg), with one gpg being the same as 17.1ppm. Soft or softened water should carry a value of less than 20ppm. In hard water areas this value may exceed 300ppm. This has a highly negative impact on the laundry process and can create significant scaling on metal pipes and surfaces.
Article courtesy of EDRO Corporation